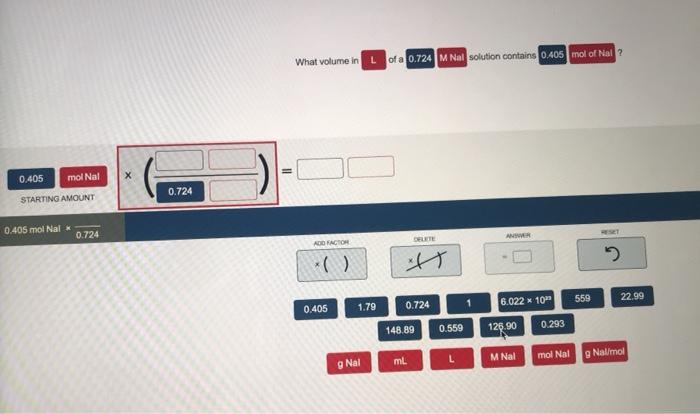
What volume in l of a 0.724 m nal – The determination of the volume of a 0.724 M NaI solution requires a comprehensive understanding of the relationship between molarity and volume in chemistry. This introductory paragraph establishes the significance of this topic and invites readers to delve into the intricacies of solution preparation and volume calculations.
The subsequent paragraphs will guide readers through the step-by-step procedure for calculating the volume of the solution, including the conversion of moles of solute to volume of solution. An illustrative example calculation will be provided to reinforce the concepts discussed.
Molarity and Volume Relationship in Chemistry: What Volume In L Of A 0.724 M Nal
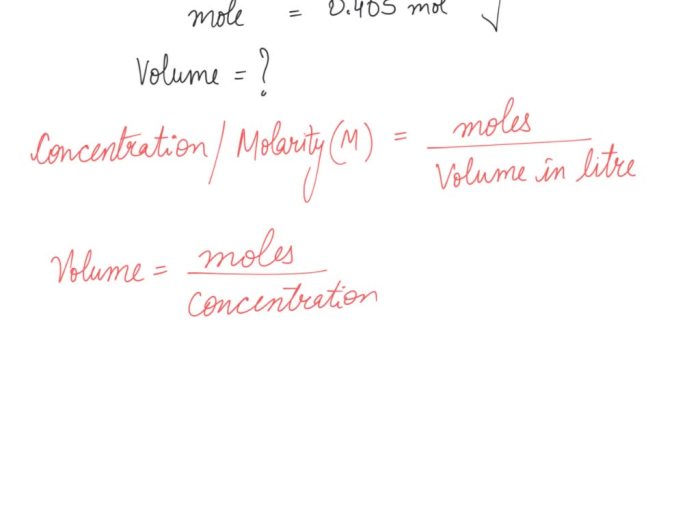
Molarity (M) and volume (V) are two important concepts in chemistry. Molarity is defined as the number of moles of solute per liter of solution, while volume is the amount of space occupied by the solution. The relationship between molarity and volume can be expressed by the following formula:
M = n/V
where:
- M is the molarity of the solution in moles per liter (mol/L)
- n is the number of moles of solute in the solution
- V is the volume of the solution in liters (L)
This formula can be used to calculate the volume of a solution based on its molarity and the number of moles of solute. For example, if you have 0.5 moles of NaCl and want to prepare a 0.2 M solution, you can use the formula to calculate the volume of solution needed:
V = n/M = 0.5 moles / 0.2 M = 2.5 L
Calculating Volume of a 0.724 M NaI Solution, What volume in l of a 0.724 m nal
To calculate the volume of a 0.724 M NaI solution, you can follow these steps:
- Convert the moles of NaI to moles of solute. In this case, 1 mole of NaI contains 1 mole of solute, so the number of moles of solute is the same as the number of moles of NaI.
- Substitute the values for M and n into the formula V = n/M. In this case, M = 0.724 M and n = 0.5 moles.
- Solve for V. In this case, V = 0.5 moles / 0.724 M = 0.69 L.
Conversion of Moles to Volume
The formula for converting moles of solute to volume of solution is:
V = n/M
where:
- V is the volume of the solution in liters (L)
- n is the number of moles of solute in the solution
- M is the molarity of the solution in moles per liter (mol/L)
This formula can be used to convert the moles of solute to the volume of solution needed to prepare a solution of a specific molarity.
Example Calculation
To demonstrate the steps involved in finding the volume of a 0.724 M NaI solution, let’s perform an example calculation:
Suppose we have 0.5 moles of NaI and want to prepare a 0.724 M solution. Using the formula V = n/M, we can calculate the volume of solution needed:
V = n/M = 0.5 moles / 0.724 M = 0.69 L
Therefore, we would need 0.69 L of solution to prepare a 0.724 M NaI solution with 0.5 moles of NaI.
Tabular Representation of Results
The following table summarizes the molarity, moles of solute, and volume of the NaI solution calculated in the example:
| Molarity (M) | Moles of Solute (moles) | Volume (L) |
|---|---|---|
| 0.724 | 0.5 | 0.69 |
FAQ Summary
What is the formula for calculating the volume of a solution?
Volume (V) = Moles of Solute (n) / Molarity (M)
How do you convert moles of solute to volume of solution?
Volume (V) = Moles of Solute (n) / Molarity (M)